- The ANMV
- Our activities
- Administrative formalities
- Our Publications
- Europe & International
- Europe
- International
- World Organisation for Animal Health (WOAH)
- International harmonisation of technical requirements for the registration of veterinary medicinal products (VICH)
- Pharmaceutical inspection cooperation scheme (PICs)
- Organisation for Economic Co-operation and Development (OECD)
- Codex Alimentarius
- Cooperation agreements
- E-learning module
Portal Veterinary medicinal products

Parallel trade in veterinary medicinal products
Parallel trade is an administrative procedure whereby a veterinary medicinal product of a common origin with a veterinary medicinal product authorised in France can be procured in another Member State of the European Economic Area under better economic conditions. The application may be filed by a wholesale distributor of veterinary medicinal products. Veterinary medicinal products subject to parallel trade are relabelled in French before being marketed in France.
This procedure does not call into question the prescription and retail conditions applicable in France.
Article 102 of the Regulation provides that, in the case of parallel trade, the wholesale distributor must ensure that the veterinary medicinal product which he wishes to obtain in another Member State shares a common origin with the veterinary medicinal product authorised in the Member State of destination.
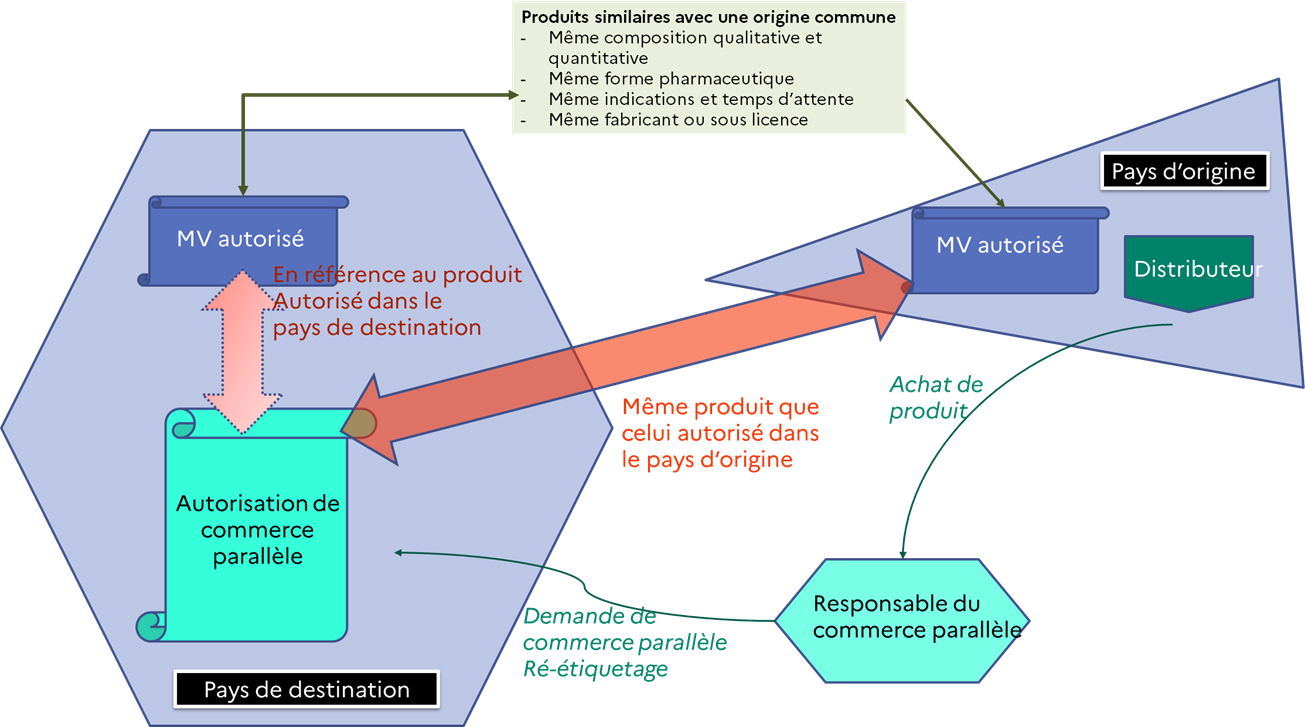
The competent authorities shall establish administrative procedures for parallel trade in veterinary medicinal products and an administrative procedure for the approval of parallel trade applications.
A wholesale distributor intending to carry out parallel trade in a veterinary medicinal product must submit a declaration to the competent authority of the Member State of destination.